
Old Earth Ministries Online Geology Curriculum© Old Earth Ministries (We Believe in an Old Earth...and God!) NOTE: If you found this page through a search engine, please visit the intro page first.
|
||
Geology - Chapter 12: WeatheringWeathering is the process of breaking down rocks, soils and their minerals through direct contact with the atmosphere. Weathering occurs in situ, or 'without movement', and thus should not to be confused with erosion, which involves the movement and disintegration of rocks and minerals by processes such as water, wind, ice, hail and gravity. |
Lesson Plan
Monday - Read Text Tuesday - Read Text Wednesday - Quiz Thursday - Review Friday - Test
|
Want more Geology? Our next curriculum is about Historical Geology. It explores the 4.5 billion year history of our planet. To view this curriculum, click here. |
|
Parents Information This lesson plan is designed so that your child can complete the chapter in five days. The only decisions you will need to make will be concerning the research task for Tuesday. It is up to you to determine if the student will simply fill in the answers, or provide a short essay answer. You will also need to determine the percentage that this research will play in the overall chapter grade, if any. |
||
|
If you have found this course useful, please consider a
donation, so that we can continue to offer
new curriculum. |
||
|
Two main classifications of weathering processes exist. Mechanical or
physical weathering involves the breakdown of rocks and soils through direct
contact with atmospheric conditions such as heat, water, ice and pressure.
The second classification, chemical weathering, involves the direct effect
of atmospheric chemicals, or biologically produced chemicals (also known as
biological weathering), in the breakdown of rocks, soils and minerals. The materials left over after the rock breaks down combined with organic material creates soil. The mineral content of the soil is determined by the parent material, thus a soil derived from a single rock type can often be deficient in one or more minerals for good fertility, while a soil weathered from a mix of rock types (as in glacial, eolian or alluvial sediments) often makes more fertile soil. Physical (mechanical) weathering Mechanical weathering is a cause of the disintegration of rocks. Most of the times it produces smaller angular fragments (like scree), as compared to chemical weathering. However, chemical and physical weathering often go hand in hand. For example, cracks exploited by mechanical weathering will increase the surface area exposed to chemical action. Furthermore, the chemical action at minerals in cracks can aid the disintegration process. Thermal Expansion Thermal expansion, also known as onion-skin weathering, exfoliation or thermal shock, often occurs in areas, like deserts, where there is a large temperature range on a daily basis. The temperatures soar high in the day, while dipping greatly at night. As the rock heats up and expands by day, and cools and contracts by night, stress is often exerted on the outer layers. The stress causes the peeling off of the outer layers of rocks in thin sheets. Though this is caused mainly by temperature changes, thermal expansion is enhanced by the presence of moisture. Freeze Thaw Weathering  Freeze thaw weathering can also be called frost shattering. This type of weathering is common in mountain areas where the temperature is around freezing point (see picture). Frost induced weathering, although often attributed to the expansion of freezing water captured in cracks, is generally independent of the water-to-ice expansion. It has long been known that moist soils expand or frost heave upon freezing as a result of water migrating along from unfrozen areas via thin films to collect at growing ice lenses. This same phenomena occurs within pore spaces of rocks. They grow larger as they attract liquid water from the surrounding pores. The ice crystal growth weakens the rocks which, in time, break up. Intermolecular forces acting between the mineral surfaces, ice, and water sustain these unfrozen films which transport moisture and generate pressure between mineral surfaces as the lens aggregates. Experiments show that chalk, sandstone and limestone do not fracture at the nominal freezing temperature of water of slightly below 0°C, even when cycled or held at low temperature for extended periods, as one would expect if weathering resulted from the expansion of water as froze. For the more porous types of rocks, the temperature range critical for rapid, ice-lens-induced fracture is -3 to -6°C, significantly below freezing temperatures. Freeze induced weathering action occurs mainly in environments where there is a lot of moisture, and temperatures frequently fluctuate above and below freezing point—that is, mainly alpine and areas around glaciers. An example of rocks susceptible to frost action is chalk, which has many pore spaces for the growth of ice crystals. Frost (Ice) Wedging Frost action, sometimes known as ice crystal growth, ice wedging, frost wedging or freeze-thaw occurs when water in cracks and joints of rocks freeze and expand. In the expansion, it was argued that since expanding water can exert pressures up to 21 megapascals (MPa) (2100 kgf/cm²) at −22 °C. This pressure is often higher than the resistance of most rocks and causes the rock to shatter. When water that has entered the joints freezes, the ice formed strains the walls of the joints and causes the joints to deepen and widen. This is because the volume of water expands by 10% when it freezes. When the ice thaws, water can flow further into the rock. When the temperature drops below freezing point and the water freezes again, the ice enlarges the joints further. Repeated freeze-thaw action weakens the rocks which, over time, break up along the joints into angular pieces. The angular rock fragments gather at the foot of the slope to form a talus slope (or scree slope). The splitting of rocks along the joints into blocks is called block disintegration. The blocks of rocks that are detached are of various shapes depending on rock structure. Pressure Release In pressure release, also known as unloading, overlying materials (not necessarily rocks) are removed (by erosion, or other processes), which causes underlying rocks to expand and fracture parallel to the surface . Often the overlying material is heavy, and the underlying rocks experience high pressure under them, for example, a moving glacier. Pressure release may also cause exfoliation to occur. Intrusive igneous rocks (e.g. granite) are formed deep beneath the earth's surface. They are under tremendous pressure because of the overlying rock material. When erosion removes the overlying rock material, these intrusive rocks are exposed and the pressure  on them is released. The outer parts of the rocks then tend to expand. The
expansion sets up stresses which cause fractures parallel to the rock
surface to form(see picture). Over time, sheets of rock break away from the
exposed rocks along the fractures. Pressure release is also known as
"exfoliation" or "sheeting";
these processes result in batholiths and granite domes.
on them is released. The outer parts of the rocks then tend to expand. The
expansion sets up stresses which cause fractures parallel to the rock
surface to form(see picture). Over time, sheets of rock break away from the
exposed rocks along the fractures. Pressure release is also known as
"exfoliation" or "sheeting";
these processes result in batholiths and granite domes.Hydraulic action Hydraulic action is when water (generally from powerful waves) rushes into cracks in the rockface rapidly. This traps a layer of air at the bottom of the crack, compressing it and weakening the rock. When the wave retreats, the trapped air is suddenly released with explosive force. The explosive release of highly pressurized air cracks away fragments at the rockface and widens the crack itself, worsening the process so more air is trapped on the next wave. This progressive system of positive feedback can damage cliffs greatly and cause rapid weathering. Salt-Crystal Growth (haloclasty) Salt crystallization or otherwise known as haloclasty causes disintegration of rocks when saline solutions seep into cracks and joints in the rocks and evaporate, leaving salt crystals behind.. As the salt crystals continually build up, they can exert more and more force upon the rock, until the rock fractures. Salt crystallization may also take place when solutions decompose rocks (for example, limestone and chalk) to form salt solutions of sodium sulfate or sodium carbonate, of which the moisture evaporates to form their respective salt crystals. The salts which have proved most effective in disintegrating rocks are sodium sulfate, magnesium sulfate, and calcium chloride. Some of these salts can expand up to three times or even more. It is normally associated with arid climates where strong heating causes strong evaporation and therefore salt crystallization. It is also common along coasts. An example of salt weathering can be seen in the honeycombed stones in sea walls. Biotic Weathering Living organisms may contribute to mechanical weathering (as well as chemical weathering, see 'biological' weathering below). Lichens and mosses grow on essentially bare rock surfaces and create a more humid chemical microenvironment. The attachment of these organisms to the rock surface enhances physical as well as chemical breakdown of the surface microlayer of the rock. On a larger scale seedlings sprouting in a crevice and plant roots exert physical pressure as well as providing a pathway for water and chemical infiltration. Burrowing animals and insects disturb the soil layer adjacent to the bedrock surface thus further increasing water and acid infiltration and exposure to oxidation processes. Another well known example of animal-caused biotic weathering is by the bivalve mollusk known as a Piddock. These animals, found 'boring' into carboniferous rocks, such as the limestone cliffs of Flamborough Head, bore themselves further into the cliff-face. Chemical Weathering Chemical weathering involves the change in the composition of rock, often leading to a 'break down' in its form. The discussion below includes the chemical formulas, for those who want to study them in depth. Specific formulas will not be testable material, although the tests may include the common names of the minerals. Dissolution Dissolution is the process of dissolving a solid into a liquid, usually with the help of an acid. Rainfall is naturally slightly acidic because atmospheric carbon dioxide dissolves in the rainwater producing weak carbonic acid. In unpolluted environments, the rainfall pH is around 5.6. Acid rain occurs when gases such as sulfur dioxide and nitrogen oxides are present in the atmosphere. These oxides react in the rain water to produce stronger acids and can lower the pH to 4.5 or even 4.0. Sulfur dioxide, SO2, comes from volcanic eruptions or from fossil fuels, can become sulfuric acid within rainwater, which can cause solution weathering to the rocks on which it falls. One of the most well-known solution weathering processes is carbonation, the process in which atmospheric carbon dioxide leads to solution weathering. Carbonation occurs on rocks which contain calcium carbonate such as limestone and chalk. This takes place when rain combines with carbon dioxide or an organic acid to form a weak carbonic acid which reacts with calcium carbonate (the limestone) and forms calcium bicarbonate. This process speeds up with a decrease in temperature and therefore is a large feature of glacial weathering. The reaction are as follows: CO2 + H2O = H2CO3 carbon dioxide + water = carbonic acid H2CO3 + CaCO3 = Ca(HCO3)2 carbonic acid + calcium carbonate = calcium bicarbonate Hydration Hydration is a form of chemical weathering that involves the rigid attachment of H+ and OH- ions to the atoms and molecules of a mineral. When rock minerals take up water, the increased volume creates physical stresses within the rock. For example iron oxides are converted to iron hydroxides and the hydration of anhydrite forms gypsum. Hydrolysis Hydrolysis is a chemical weathering process affecting silicate minerals. In such reactions, pure water ionizes slightly and reacts with silicate minerals. An example reaction: Mg2SiO4 + 4H+ + 4OH- = 2Mg2+ + 4OH- + H4SiO4 olivine (forsterite) + four ionized water molecules = ions in solution + silicic acid in solution This reaction results in complete dissolution of the original mineral, assuming enough water is available to drive the reaction. However, the above reaction is to a degree deceptive because pure water rarely acts as a H+ donor. Carbon dioxide, though, dissolves readily in water forming a weak acid and H+ donor. Mg2SiO4 + 4CO2 + 4H2O = 2Mg2+ + 4HCO3- + 4H4SiO4 olivine (forsterite) + carbon dioxide + water = Magnesium and bicarbonate ions in solution + silicic acid in solution This hydrolysis reaction is much more common. Carbonic acid is consumed by silicate weathering, resulting in more alkaline solutions because of the bicarbonate. This is an important reaction in controlling the amount of CO2 in the atmosphere and can affect climate. Aluminosilicates when subjected to the hydrolosis reaction produce a secondary mineral rather than simply releasing cations. 2KAlSi3O8 + 2H2CO3 + 9H2O = Al2Si2O5(OH)4 + 4H4SiO4 + 2K+ + 2HCO3- Orthoclase - aluminosilicate feldspar + carbonic acid + water = Kaolinite - a clay mineral + silicic acid in solution + potassium and bicarbonate ions in solution Oxidation Within the weathering environment chemical oxidation of a variety of metals occurs. The most commonly observed is the oxidation of Fe2+ (iron) and combination with oxygen and water to form Fe3+ hydroxides and oxides such as goethite, limonite, and hematite. This gives the affected rocks a reddish-brown coloration on the surface which crumbles easily and weakens the rock. This process is better known as 'rusting'. Biological A number of plants and animals may create chemical weathering through release of acidic compounds. The most common form of biological weathering is the release of chelating compounds, that is acids, by trees so as to break down elements such as aluminum and iron in the soils beneath them. Once broken down, such elements are more easily washed away by rainwater. This process exists as metals such as iron can be toxic and hinder the a tree's growth. Extreme release of chelating compounds can easily affect surrounding rocks and soils, and may lead to podsolisation of soils. Building Weathering Buildings made of limestone are particularly susceptible to weathering. Weeds grow almost anywhere without many problems. They can sometimes germinate in the gutters of buildings where they have been transported to by the wind. As they proceed to grow they plant their roots down into the rock that the building is made up of forcing their way further down. This causes the rock to exfoliate over a long time, small fragments crumbling away now and then. Statues and ornamental features can be badly damaged by weathering, especially in areas severely affected by acid rain which is caused by pollutants put into the air. The Importance of Joints in Weathering
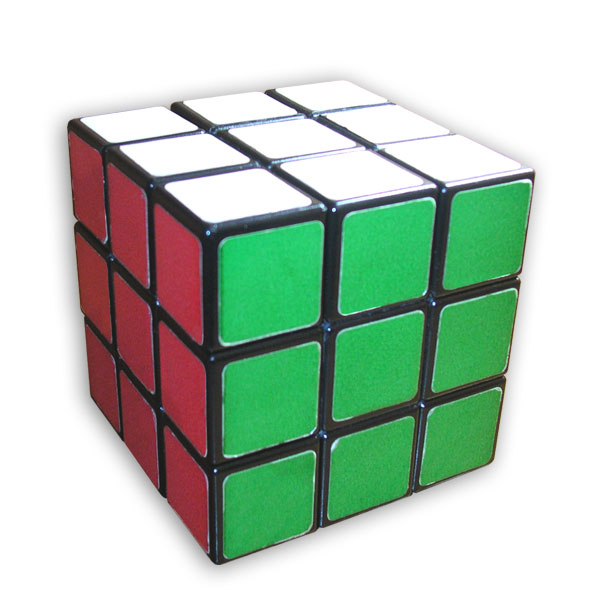
The process of jointing greatly increases the amount of surface space
exposed to weathering. To visualize this process, consider the Rubik's
Cube (picture at right).
Imagine that the white surface is the earth's surface. If this cube were a solid block of rock, then only the white surface would be exposed to weathering. Let's imagine that the entire cube is 3 meters across. Therefore the entire surface area exposed to weathering is 9 cubic meters.
If joints develop from weathering, so that each
colored face is exposed (the outer surfaces), then the total surface now
exposed to weathering would be six times greater, or 54 cubic meters.
If further joints develop, one meter apart, breaking the rock into three
sections across and three sections wide, and three sections deep, we now
have 27 smaller cubes, with all their surfaces exposed to weathering.
This would increase the total surface area exposed to weathering to 162
square meters.
The joints in the rock provide a system of channels through which water can move, leading to more mechanical and chemical weathering. This process can occur on the surface, or even hundreds of meters beneath the earth's surface. The image at right shows an aerial photo of a large rock body at Arches National Park that is cut by many joints.
The Products of Weathering
If you have ever examined dirt, then you have examined the products of
weathering. As The upper layer of the regolith is known as soil. Soil is further divided into divisions known as horizons, which are distinguished by composition, color, and texture. Rocks typically weather into rounded surfaces. This is the result of weathering occurring on all sides at one time. This tendency is known as spheroidal weathering. A special type of spheroidal weathering is known as exfoliation, which was discussed above.
Climate and Weathering
Weathering is a direct result of the climate. The climate will determine the rate and type of weathering that occurs to a rock. The greatest weathering agent is water. The amount of precipitation in an area determines the amount of weathering that can occur, although other factors also play a part, such as the intensity of the rain, rate of evaporation, water drainage, and water infiltration into the soil. Tropical, humid environments produce the thickest layers of soil. Because of the high precipitation rate, and high temperatures, chemical weathering can occur rapidly, developing soils with depths of greater than 70 meters. In more arid regions, the soil may be completely absent, with the bedrock exposed. In polar climates, the soils is very thin, because it is too cold for much chemical weathering to occur. The rate of weathering depends upon three main factors.
1. The minerals being weathered and their resistance to weathering 2. The climate 3. The amount of surface area exposed
Minerals can have varying degrees of resistance to weathering. Typically, minerals that crystallize at a high temperature weather the easiest. Climate makes a large impact. For example, in Hawaii, fresh lava flows will weather enough in a few years, and can support vegetation. Lava flows with the same composition in other, less humid environments, have remained unweathered since the ice ages ended over 11,000 years ago.
Scientists can use known rock surfaces to measure weathering rates.
For example, Today you will complete an 10 question practice quiz. The link to the quiz will open a new window. You can come back here and check your answers. Do not click the Back button on your browser during the quiz. After the quiz, continue your research project, if necessary. Please review the terms in bold in the text, and ensure you have completed your research work from Tuesday. Today you will take the end of chapter test. Please close all other browser windows, and click on the link below. During the test, do not click on the Back button on your browser. Return to the Old Earth Ministries Online Geology Curriculum homepage. NOTE: Most of the text and pictures for this lesson come from Wikipedia
|

 rocks weather, they form what is known as regolith,
which is the loose soil that covers the earth's bedrock. You can see
the transition of rock to regolith in road cuts or stream valleys. The
image at left shows the regolith on top of bedrock. The fractured
rocks at the bottom of the regolith are part of the regolith. As
joints (fractures) progress downward, the top of the bedrock erodes, and the
base of the regolith moves deeper.
rocks weather, they form what is known as regolith,
which is the loose soil that covers the earth's bedrock. You can see
the transition of rock to regolith in road cuts or stream valleys. The
image at left shows the regolith on top of bedrock. The fractured
rocks at the bottom of the regolith are part of the regolith. As
joints (fractures) progress downward, the top of the bedrock erodes, and the
base of the regolith moves deeper.  buildings, tombstones, and monuments have known dates of construction, and
their rock surfaces can be examined for erosion. A great example of
this is the pyramids of Egypt. The pyramid at right has visible debris
on each step, which comes from the erosion of rocks that make up the
pyramid.
buildings, tombstones, and monuments have known dates of construction, and
their rock surfaces can be examined for erosion. A great example of
this is the pyramids of Egypt. The pyramid at right has visible debris
on each step, which comes from the erosion of rocks that make up the
pyramid.